About the group
Our group studies the interaction between the oral microflora and the immune system and the pathogenesis of oral lichen planus and periodontitis. The group is composed of basic science researchers and people with dental education.
Projects
The aims of our projects are (1) to elucidate mechanisms behind the interaction between the oral microflora and other antigens present or passing through the oral cavity, and the host, and (2) investigate the pathogenesis of oral lichen planus and periodontitis.
Antigen presenting cells in the oral cavity
The oral cavity is the port of entry for food and is also incessantly exposed to pathogenic and commensal microbes by which it is populated. The deeper oral tissues are physically protected by the epithelium and the underlying lamina propria and chemically against infection by compounds released by epithelial cells and secreted in saliva. A great number of immune cells reside in the epithelium and in the lamina propria where they limit penetration and invasion by pathogens and commensals by eliciting inflammatory responses.
For the host, however, it is also crucial that food and commensals that reside in their niche are immunologically tolerated, both within the oral cavity and elsewhere in the body. Peripheral immune tolerance is now acknowledged as an active process in which either T-cell anergy or suppression of T-cell responses by Tregs or other regulatory cells are induced. Despite the fact that the oral cavity is the first site to be exposed to food and that the oral mucosa is colonized by microorganisms soon after birth, little is known about its role in the development of tolerance (1). The immune system in the oral cavity is part of the body’s mucosal immune system that also covers the gastrointestinal (GI), respiratory and urogenital tracts and the exocrine glands. While the composition and function of the immune system in the gut is well outlined, this is less well understood in the oral cavity (2).
It is thought that the oropharyngeal compartment, just like the gut, comprises inductive and effector sites. At inductive sites, antigen-presenting cells (APCs) capture antigen and migrate to the regional lymph nodes. Here, they present antigen to naive T and B cells which subsequently differentiate and proliferate into B and T effector lymphocytes. Alternatively, APCs may induce tolerance. If induced in the gut, for example, the effector lymphocytes are “primed”, instructed, to migrate back to effector sites in the local mucosa of the same body site, as well as to distal mucosal sites. The latter is part of the concept of a “common mucosal immune system” (CMIS) in which exposure of a specific antigen at one mucosal site elicits responses that protect both local and distant mucosal sites. The homing to mucosal or other sites is mediated by the expression of specific adhesion molecules on the effector lymphocytes.
The predominant effector sites in the oral cavity are the salivary glands where SIgA is exported as the most abundant immunoglobulin in saliva. Little is, however, known about the nature and localization of inductive sites that are most important for B-cell activation, migration and subsequent SIgA-secretion in saliva. Recent studies indicate that the nasopharynx-associated lymphoid tissue (NALT), which is constituted of the adenoids and palatine tonsils in the human, may have a prominent function in the generation of IgA-producing plasma cells that migrate to the salivary glands. The relative contribution may vary between the different salivary glands (2, 3). More knowledge regarding the inductive mechanisms as well as the homing mechanisms that decide the location to where the effector cells are destined will contribute to elucidate the composition and function of the system (3).
Dendritic cells (DCs) constitute a unique cell population in the immunity of the surface barriers in the body. The conventional dendritic cell “paradigm” involves the dendritic cell residing in epithelial and subepithelial tissues capturing antigens, migrating to regional lymph nodes where they present antigen to naive T and B lymphocytes with the subsequent differentiation, proliferation and migration of effector lymphocytes back to peripheral tissue where they conduct their effector functions (4). The different types and combinations of pattern recognition receptors on the DC surface as well as their anatomical locations and the influence by the local cytokine milieu are important for generating immune responses that are adjusted to the level of threat (1).
Hence, by orchestrating the T- and B-cell response to a diverse population of foreign- and self antigens, DCs play a major role in deciding if the resulting immune responses are immunogenic or tolerogenic (1). The potential for exploiting DCs for therapeutic purposes, e. g. in vaccines and in sublingual immunotherapy (SLIT), is an ongoing process in both academia and the industry (3, 5).
The refinement of flow cytometric techniques, lineage tracing and genomic profiling over the recent years have leaded to an understanding of DCs as a heterogeneous group of cells, with deviant origins, anatomical locations and function.
Current understanding of human DC heterogeneity stems from studies of skin, intestinal and blood DCs. There is little knowledge on the distribution and different subclasses of dendritic cells in human oral mucosa, but studies in mice indicate a great diversity in subclasses of DCs, both qualitatively and quantitatively between different loci in the oral cavity (6). In general, DCs are now typically sectioned into 3 main groups; conventional DCs (cDCs), monocyte-derived DCs (mDCs) and plasmacytoid DCs (pDCs). Langerhans cells (LCs) represent a unique APC that is described as an intermediate between DCs and macrophages, based on phenotype and immunoregulatory properties. Further subdivisions, differences between mice and human subtypes as well as changes of phenotypes due to spontaneous maturation add increasing complexity to the system.
In this project, we examine how APCs in the oral cavity interact with commensals.
Resolution of inflammation
Inflammation is an essential biological process of the immune host response, which is activated when tissue natural homeostasis is disturbed after infection or injury. By design, the inflammatory process is programmed to cease, allowing the return of the affected tissue to its pre-inflammatory state and function. In the past, it was believed that inflammatory resolution was a passive event which results upon the dilution of chemokine gradients over time, thus reducing the chemotaxis of leukocytes to the site of injury. However, evidence from studies performed the last few decades has shown that it is a carefully orchestrated active process (Headland & Norling 2015). Specifically, it is accomplished through a sequence of over-lapping events during which pro-inflammatory mediators signal the generation of pro-resolving mediators or induce their receptor targets. Thus, the peak of the acute inflammatory response is considered to be the beginning of resolution, suggesting a concept where the “beginning programs the end” (Perretti 2015).
Several endogenous specialized pro-resolving lipid-derived mediators (SPMs) have been recently identified. Resolvins (resolution-phase interaction products) are enzymatically generated from essential omega-3 polyunsaturated fatty acids (PUFAs). Within this family two molecular series of resolvins have been characterised, namely E- and D- series resolvins which present with distinct structural and biochemical properties.
Acting either as agonists or antagonists at specific receptors (CMKLR1, ChemR23, BLT1, ALX/FPR2 and GPR32), resolvins can elicit a spectrum of cell type specific responses that collectively block the inflammatory process (Uddin & Levy 2011). Critical prerequisites for inflammation to switch off are the removal of the inciting stimulus, inhibition of leukocyte trafficking, pro-inflammatory mediator catabolism, phagocytosis of apoptotic cells and clearance of the lesion (Gilroy & De Maeyer 2015). However, for reasons that are not yet fully elucidated, one or more of the above mentioned pro-resolving pathways might be insufficiently engaged, allowing for the acute inflammatory response to continue undisturbed and to result in chronic pathology.
Periodontitis is a biofilm-induced chronic inflammatory disease, which results in loss of periodontal connective tissues and alveolar bone support around the teeth. Long-term studies have shown that untreated periodontal disease may result in tooth loss (Löe et al. 1986) Historically, it was believed that periodontal inflammation was mainly driven by the bacterial influx. However, it is now beyond doubt that besides the role played by periodontopathogens, it is the host inflammatory response to the microbial challenge which initiates and establishes the degradation of the periodontium (Hajishengallis 2014). In fact, although intended for host defense against invading microbes, leukocytes can amplify tissue destruction via the release of pro-inflammatory mediators, reactive oxygen species (ROS) and enzymes (Serhan 2010). The pathogenesis of the disease is therefore characterized by the host’s immune cell-mediated self-destruction of tissues in response to the present microflora (Hasturk et al. 2006).
Periodontal homeostasis can be disrupted by a variety of host- or microbe-related factors. Under this category fall congenital or acquired host immunodeficiencies, immunoregulatory defects associated with mutations or polymorphisms, old age systemic diseases, environmental factors, epigenetic modifications in response to environmental changes and the presence of periodontitis associated microbiota that can convert a symbiotic environment to a dysbiotic one (Hajishengallis 2014). Susceptibility to periodontitis as well as persisting inflammation might be due to the presence of one or a combination of the above mentioned factors, which are able to cause dysbiosis of the periodontal microbiota and progression to chronic periodontal disease. In this direction, an inadequate pro-inflammatory or pro-resolving host response, or an imbalance between the two is a clear mechanism explaining the pathogenesis of the disease. Hence, controlling the fate of the pro-resolving pathways could have an impact on the conversion of a gingivitis case to periodontitis (Van Dyke 2007). During the last decades, several studies investigating the role of resolvins and especially RvE1 in periodontitis have provided a window to explore the pathophysiology of this chronic inflammatory disease.
In this project, we study the role of resolvins in periodontal inflammation.
Pathogenesis of oral lichen planus (OLP)
Oral lichen planus is a relatively common chronic inflammatory disease affecting between 0,2 and 2% of the population. Despite considerable research, the cause of lichen planus remains unknown. CD8+ T cells are recruited to the tissue by an unknown mechanism, and basal keratinocytes are induced to undergo apoptosis.
We have done several investigations over the years on the pathogenesis of OLP. Currently we are focusing on two projects.
Langerhans cells in OLP
It is well established that Langerhans cells (LCs) are increased in the epithelium of oral lichen planus lesions. Still, their function in the disease initiation and -development remains to be elucidated.
Langerhans cells are the only antigen presenting cell type that resides outermost in the epithelium of surface-facing organs. With a density of approximately 700 LCs/mm2 in the epidermal layer of the skin, LCs constitute 3-5 % of the cells in the epidermis (9). In the oral mucosa, there are fewer LCs pr/mm2 compared to skin, but they are more abundant since the epithelium of the oral mucosa is thicker than skin.
Despite that they have been known for over 100 years, Langerhans cell function and ontogeny was for a long time poorly understood. Extensive research, mostly of skin LCs, the in recent years have revealed å complex an multifunctional cell type that are able to customize to the environment and levels of threat. Based on function, Langerhans cells were for a long time classified as dendritic cells. Like conventional dendritic cells, LCs have a migratory capacity that permits display of antigens to naive T-cells in regional lymph nodes. In skin, bacteria-primed LCs are shown to have a poor capacity to internalize bacteria and present antigen on MHC-II with a consequently poor ability to induce specific T-cells. Instead they can stimulate the development of Foxp3+ Tregs that inhibit proliferation of T-cells that are specific for the same antigens. Via Treg-activation, LCs appear to have a protective role against contact dermatitis and experimental autoimmune encephalomyelitis.
The recent years, however, a leading trend in the field of immunology is to classify immune cells based on ontogeny. Recent studies have revealed that skin LCs originate from yolk sac-derived myeloid precursors and are recruited to the epithelium of peripheral non-lymphoid tissue in early fetal development where they adapt a macrophage-like morphology. In contrast to conventional Dendritic cells, they under steady-state conditions constitute a self-renewable population without the constant need for replacement by bone marrow-derived precursors. The leading trend in the field is therefor to define skin Langerhans cells as Macrophages. Langerhans cells residing in the oral mucosa (oLCs), are far less understood than their skin counterpart, and most studies of ontogeny and function is from mice. Recent studies conclude that mice oLCs, in contrast to skin LCs, are not a radioresistant, self-renewing population, but derived from blood monocytes with a short half time in the tissue. Based on the ontogeny classification, it is tempting to classify mice oLCs as dendritic cells. The ontogeny of human oLC is so far not known, and little is known about their life span in oral mucosa.
Knowledge of the life span of Langerhans cells can be crucial for the understanding of graft versus host disease, affecting a group of patients after bone marrow transplantation. Self renewable, long lived skin LCs are thought to provide a continuous source of host antigen to donor derived T-cells. Since graft versus host disease often affects the oral mucosa, showing oral lichen planus-like lesions, knowledge of Langerhans cell lifespan is of crucial importance.
As a consequence of the prior classification of LCs as DCs, the research focus has been on their DC-like functions. Their role in naïve-T cell activation in regional lymph nodes have been extensively studied, but less is known about their macrophage-like functions locally in the tissue. A study using only autologous human tissue show that steady state skin Langerhans cell induce tolerance by activation of local resident memory T regulatory cells (Tregs). However, in the case of pathogen challenge, the same cells will rather activate local resident effector memory T cells.
Here, we study LCs in OLP lesions.
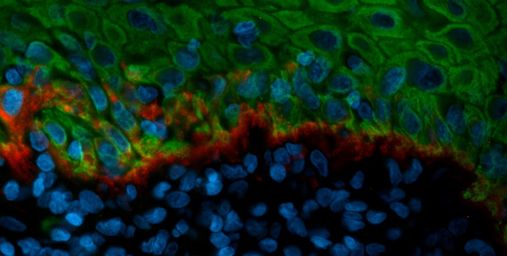
Disturbances of hemidesmosomes in OLP
Hemidesmosomes (HD) link basal oral keratinocytes to the basal membrane. This anchorage is physically important but is also an important structure regulating different important cellular functions, e.g. cell polarity.
HD are known to be disturbed in OLP. In this study, we examine the fine molecular details of such disturbances using different in situ methods and cell culture.
Cooperation
- Bruno Loos, ACTA, University of Amsterdam
- Zlatko Dembic, IOB
- Tine Søland, IOB
- Kine Marita Knudsen Sand, OUS